Chemical Management Software
Centralize your chemical data for streamlined management, improved safety and minimized risk.
Centralize your chemical data for streamlined management, improved safety and minimized risk.
Our Chemical Management software also helps businesses control everything from raw materials to final product packaging, supporting precise, predictable and scalable chemical programs for today’s ever-changing regulatory landscape and global supply chain.
By centralizing your chemical data, you can improve your reporting abilities, easily access Safety Data Sheets from any site, prescreen materials before they arrive on site and much more.
Every product steward knows the importance of accurately tracking and reporting the materials on their sites. Having the right tool in place can reduce the burden. And the right system can eliminate your fears of non-compliance, wasted resources and inefficiencies. Plus, increased transparency allows you to provide accurate reports to stakeholders.
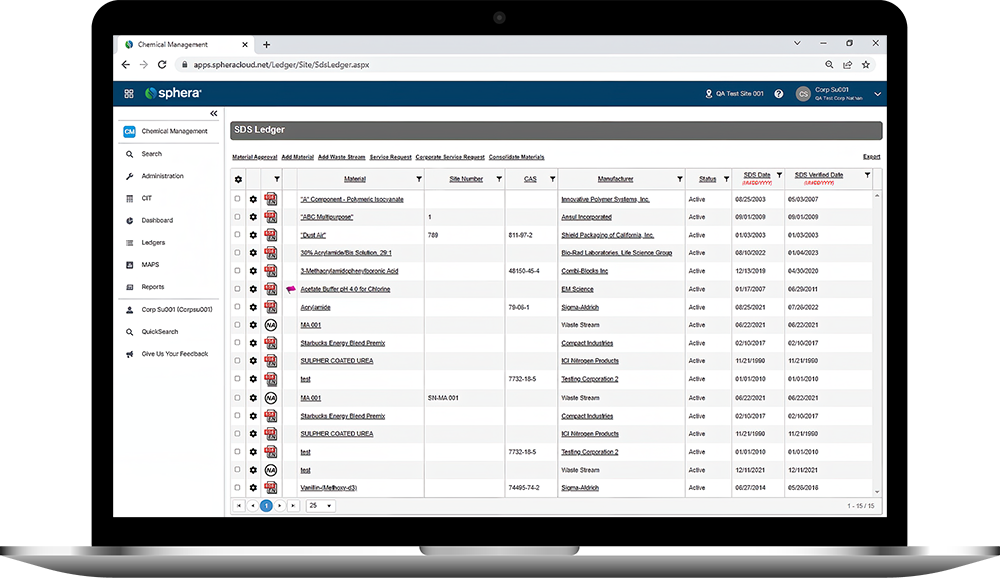
Sphera’s Safety Data Sheet Management software enables organizations to ensure universal access to Safety Data Sheets (SDS), manage the physical arrival and departure of chemicals on site and easily report on all of their chemical data.
Sphera’s Chemical Inventory Management software makes it easy to track inventory information and maintain compliance with regulatory requirements.
Country by country, you can access tools and services to manage recycling requirements for electronic waste (WEEE), batteries and packaging around the world.
Find out why industry leaders choose SpheraCloud for Chemical Management.
Centralized and standardized critical safety information across the chemical lifecycle to generate efficiencies.
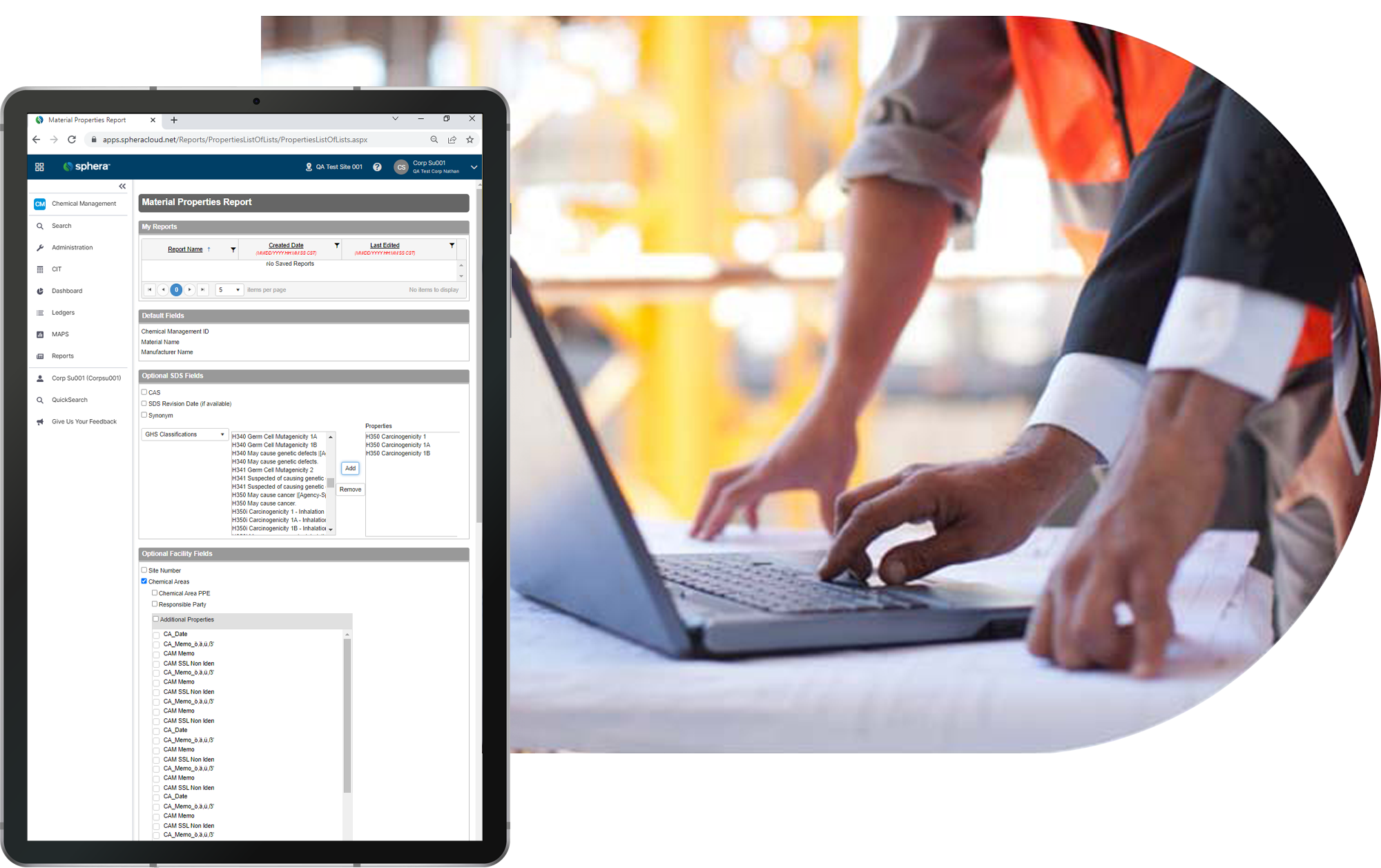
Provide fast access to up-to-date SDS and chemical safety documentation. Comply with right-to-know requirements and get the data your team needs, when they need it most.
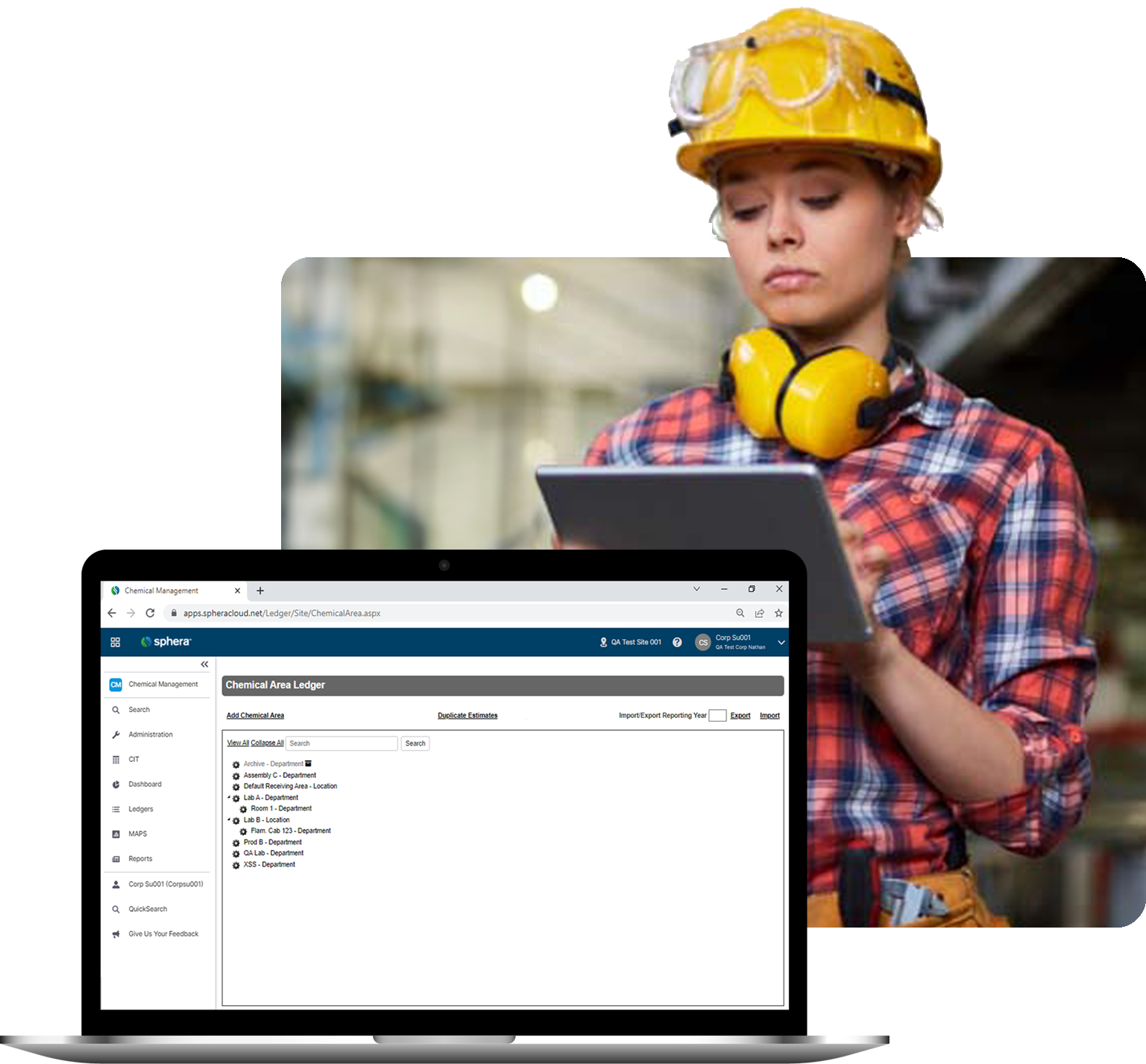
Easy reporting on anything at any time keeps suppliers, customers and regulatory agencies informed.
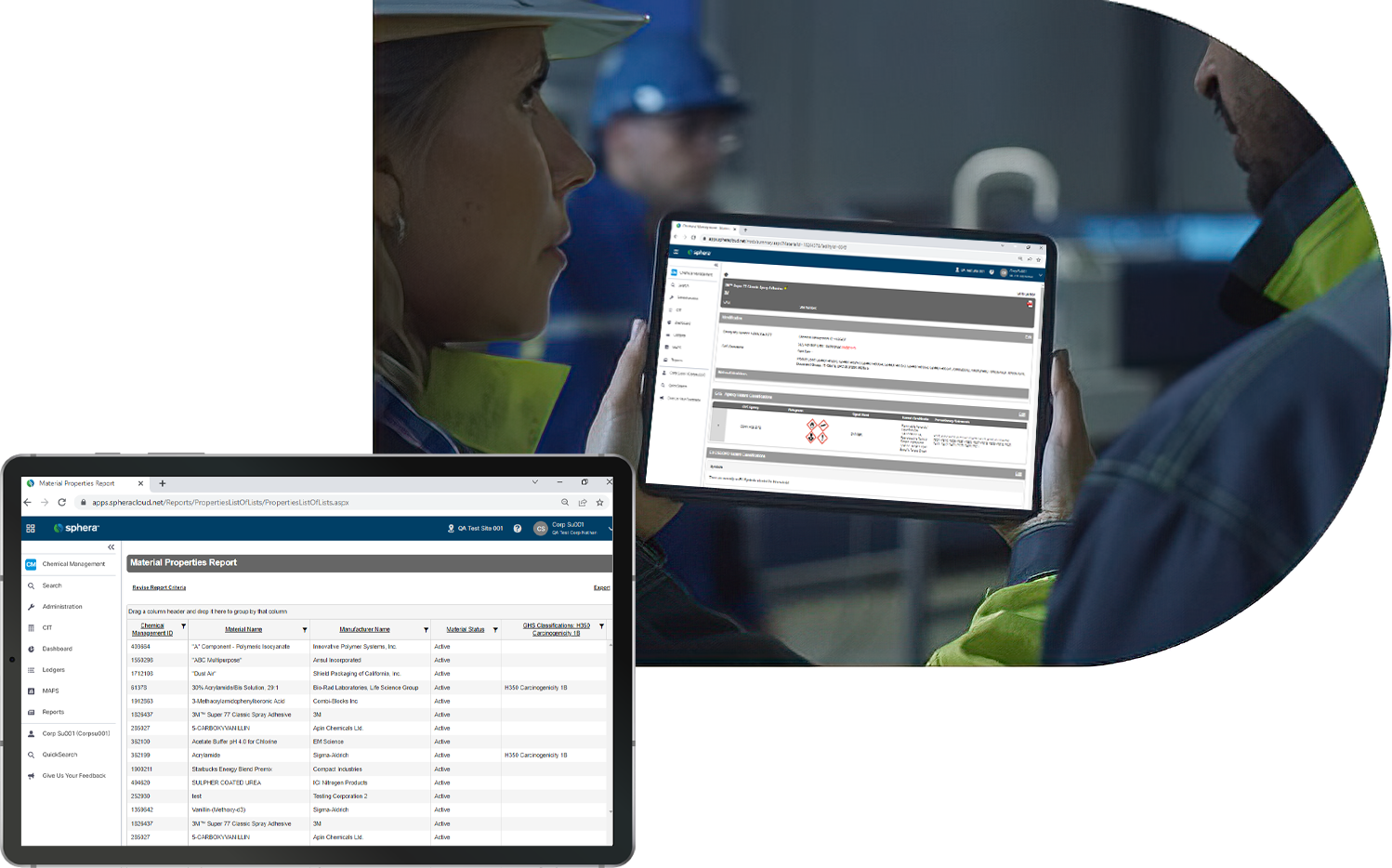
Leverage insights on hazardous chemical data and how they align workflows and mandates.
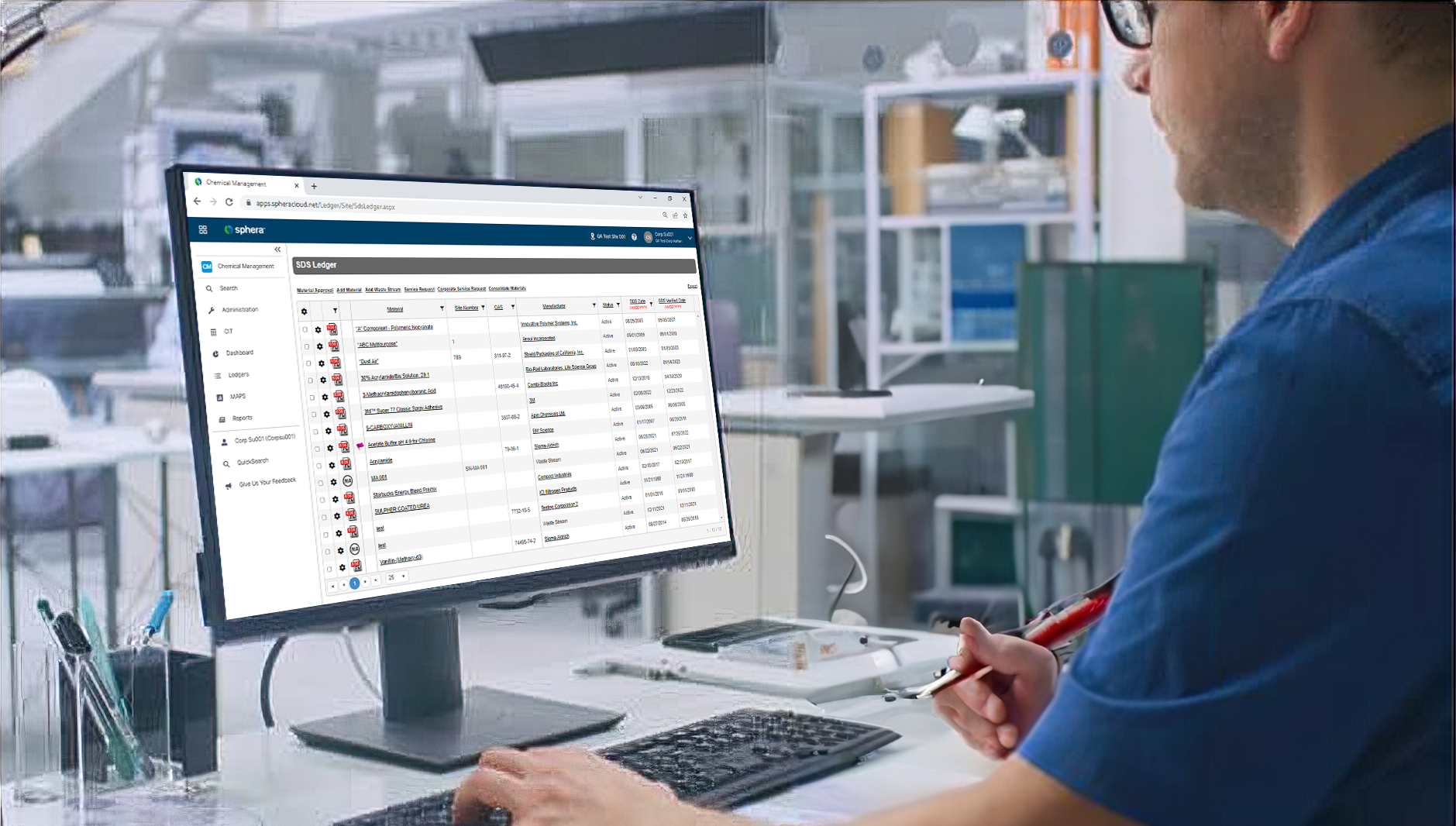
Providing product stewards with the tools they need to take total control of chemicals.
Centralize Safe Data Sheets so employees can quickly assess critical safety information on the job.
Keep track of hazardous substances as they move through your facilities, from pre-arrival to departure.
Create organization-specific workflows for approval processes, saving time and improving operational efficiency.
Keep up-to-date on local, regional and international guidelines to ensure you’re always compliant.
Large and small businesses trust SpheraCloud to optimize their Product Stewardship.
Engage with our robust content library.
Learn more about what SpheraCloud can do for your chemical management program.